- Accueil
- Centre De Connaissances
- Film Formation in Polymer Coatings
Film Formation in Polymer Coatings
An overview of film formation process
Film formation is the process by which a thin, continuous polymer layer develops on a substrate after application of a liquid coating. In paints, inks and other surface coatings, this defines the functional protective and aesthetic performance. Depending on the technology, film formation may occur by solvent (or water) evaporation from a polymer solution/ dispersion, by the melting of solid polymer particles, or by in-situ polymerisation/ curing. Coatings range from traditional solvent borne to water borne dispersions and high solids or powder coatings, but in all cases a defect-free continuous film is required for protection, appearance and durability. This is critical in industrial coatings (architectural, wood finishes etc.) where the coating must seal, resist weathering and meet regulatory requirements (e.g. low VOCs).
For example, modern regulations strongly favour low-VOC formulations and industry reports note an accelerating shift to water borne coatings (now >50% of major markets) despite some performance trade-offs (marketsearchfutue, 2025). Film formation underlies all these products and understanding its mechanisms and controlling factors is crucial to formulating high-performance coatings.
Mechanisms of Film Formation
There are two broad classes that describe film formation:
Solution (solvent-borne) systems: here the polymer is dissolved in a volatile solvent. After application (spraying, dipping etc.), solvent evaporation simply causes the polymer chains to collapse into a solid film. “For solutions, film formation occurs as the solvent evaporates, since the polymer chains are intimately mixed” – Felton (Researchgate, 2013). In practice, the polymer precipitates onto the substrate as the solvent leaves; additives may aid wetting, but no coalescence of separate particles is required if the polymer was fully dissolved.
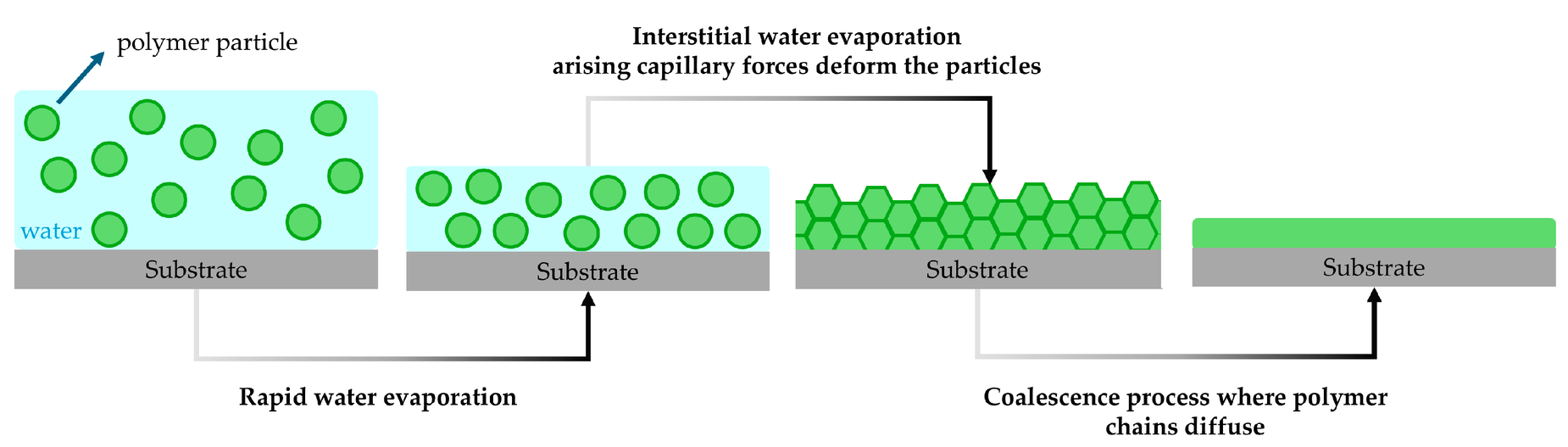
Dispersion (latex or emulsion) systems: in waterborne coatings the polymer is supplied as an aqueous dispersion of solid particles (latexes). Film formation then involves (a) water evaporation, (b) close-packing and deformation of the polymer particles, and (c) coalescence by interdiffusion of the polymer chains. Initially, water removals brings the spherical particles into contact; continued drying and capillary forces cause the particles to deform and pack tightly (Laven, 2001). Crucially, polymer chains must migrate between particles to heal gaps– this interdiffusion is called coalescence.
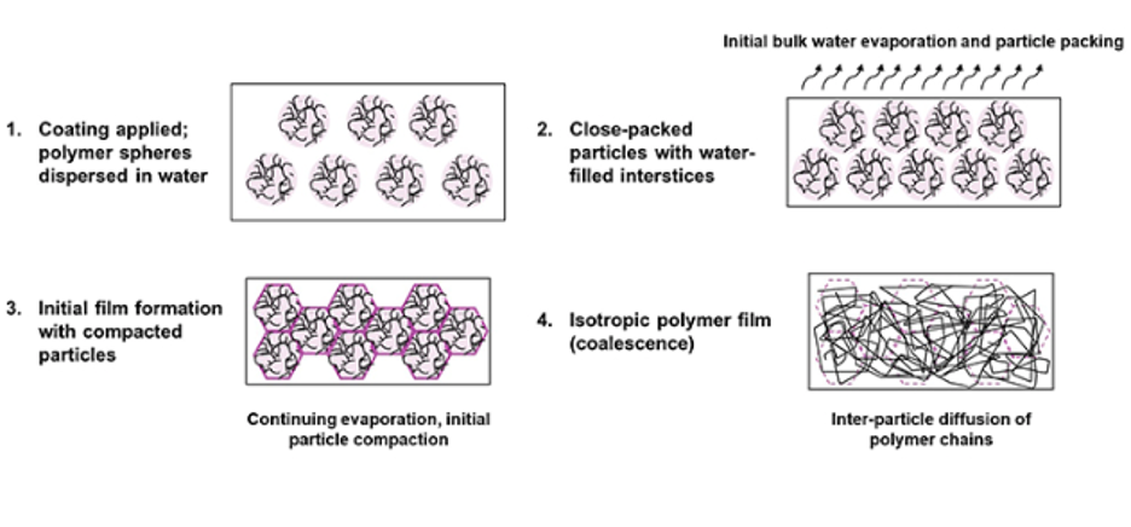
Polymer latex film formation… occurs as particles pack as water evaporates. Continued evaporation causes the particles to pack and deform; film coalescence is only achieved when inter-particle diffusion of polymer chains occurs.
Ingrid K. Meier, 2022.
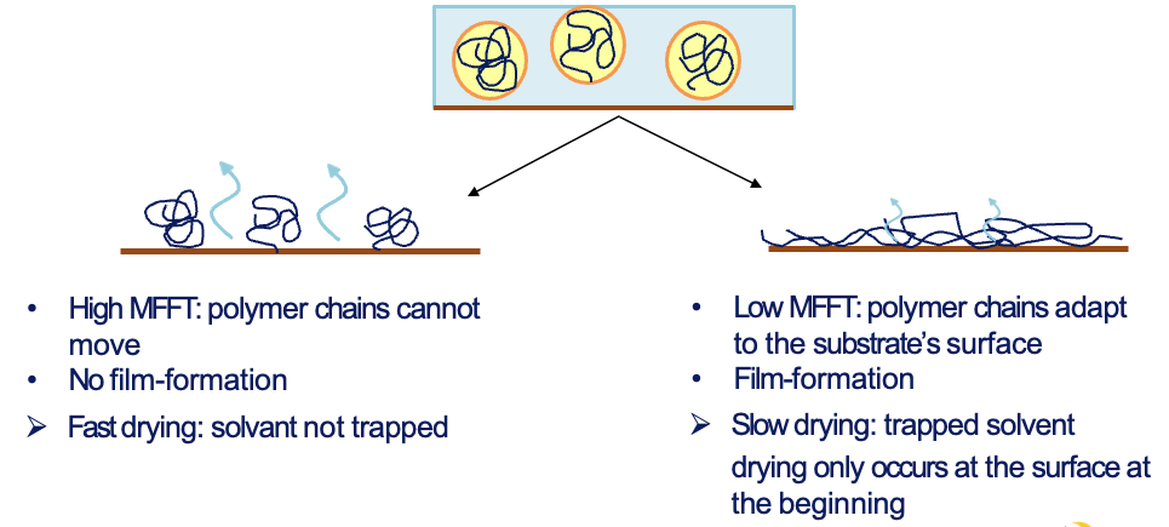
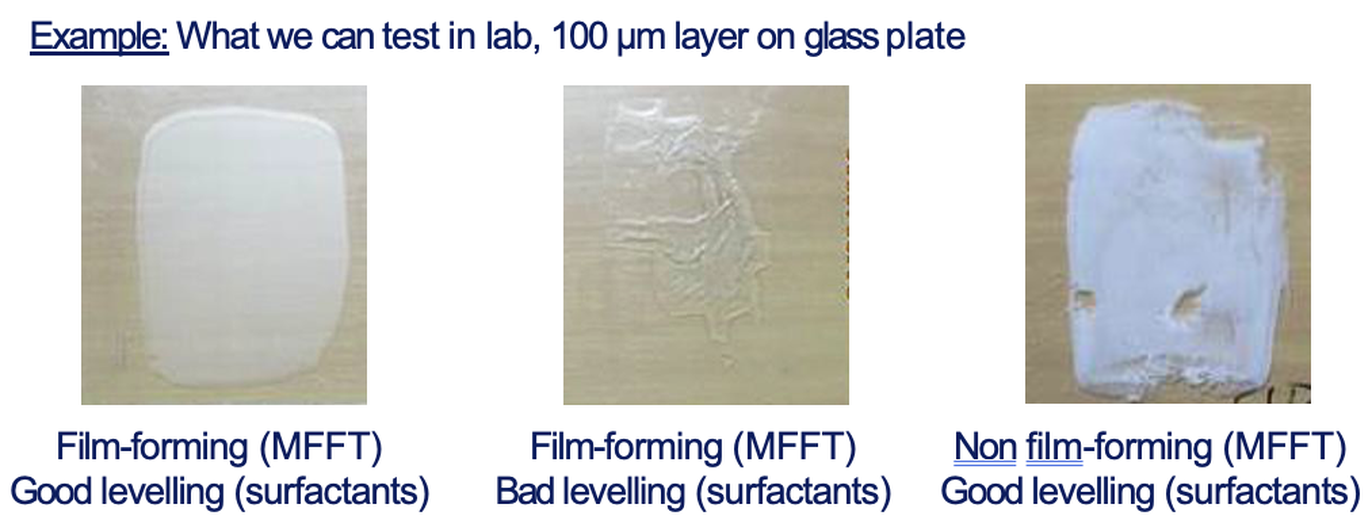
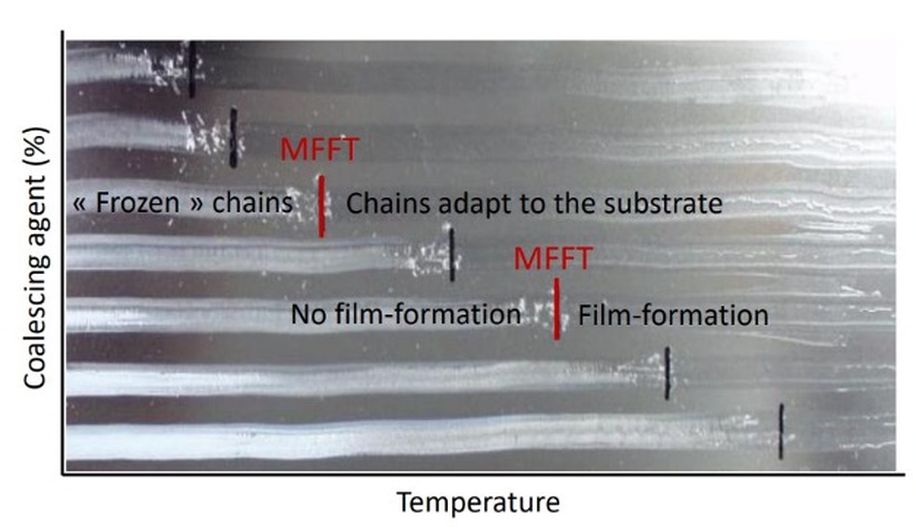
Below a certain temperature particles may remain only partially fused, yielding cracked films. This critical threshold is the minimum film-forming temperature (MFFT). Drying above the MFFT allows a continuous film to be formed (coatingsworld, 2022)
Factors Influencing Film Quality
Many variables control whether a coating forms a uniform film and its final properties. Key factors include polymer properties, additives and environmental/ substrate conditions:
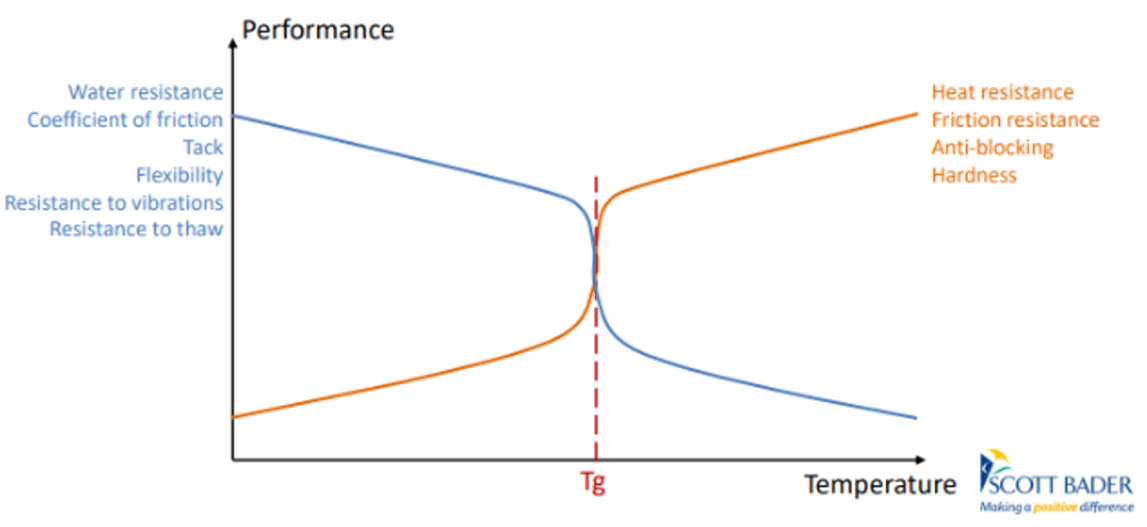
Glass transition and MFFT
The polymer’s glass transition temperature (Tg) is central. If Tg Is below application temperature, particles or droplets can readily coalesce without added aids. In contrast, high Tg polymers may be glassy at room temperature, so particles cannot fuse unless the temperature is raised, or coalescing agents are added. The MFFT corresponds roughly to Tg (often a few degrees below). Operating below MFFT yields a powdery or cracked film; adding plasticisers or coalescing solvents temporarily lowers the effective Tg and MFFT, enabling film formation. For example, common coalescents like tripropylene glycol butyl ether lower polymer Tg and MFFT significantly, whereas more water-soluble co-solvents (propylene glycol) are less efficient (coatingsworld, 2022).
Polymer chemistry and architecture
Chemistry (e.g. acrylic vs epoxy) affects chain mobility, crosslink density and interactions. Copolymer composition, molecular weight and particle morphology (core-shell structures) all influence coalescence. A core-shell latex may have a hard core and softer shell to balance hardness and film ability. Texicryl 13-601 Polymer molecular weight and branching alter viscosity and surface tension. Dissolved films (high-solids solutions) may suffer from differential evaporation if solvents have varying volatility.
Additives and solvents
Wetting agents and surfactants help the liquid coating spread uniformly; poor wetting can cause “de-wetting” (pinholes) on low-energy substrates. Rheology modifiers ensure sag-resistance without hindering levelling. Pigments and fillers affect flow and can trap solvents or plasticisers; titanium dioxide, for instance, increases viscosity. Often, coalescing agents are used in water borne systems to ensure film formation at ambient temperature. These are typically volatile solvents added at a few percent: as they evaporate after film formation they raise Tg again. The balance of coalescent content is critical – too little gives incomplete fusion, too much can soften the film or cause VOC issues. In low VOC formulations, novel multifunctional “coalescing surfactants” have been developed that simultaneously reduce surface tension and MFFT with minimal volatility.
Drying conditions (temperature, humidity)
Temperature must exceed MFFT for complete film formation. Higher drying temperature accelerates solvent loss and polymer flow. Humidity plays a surprisingly small role in latex film formation: studies show that “RH virtually does not influence” the forces that drive particle coalescence (Laven, 2001). In fact, moderately high RH (relative humidity) can slightly aid coalescence by plasticizing the polymer with water, but the effect is usually modest. Drying at low temperatures (below MFFT) leaves a white, scattering film full of voids and weak adhesion.
Substrate factors
The surface energy and cleanliness of the substrate affect film formation. A clean, high-energy surface (metal or primed wood) promotes rapid wetting and adhesion. Low energy or contaminated substrate may cause incomplete wetting or early de-wetting; this can lead to pinholes or uneven drying. In powder coatings, substrate preheating in often used to improve wetting of the molten powder.
Sustainable and High-Performance Coatings
R&D is focusing on combining sustainability with performance. As summarized by Pieters and Mekonnen (2024), polymeric coatings “have shifted towards waterborne, minimising harmful emissions…Waterborne coatings have seen substantial uptake (e.g. architectural, automotive). High‐solids waterborne systems (60–70% solids) are now common in industrial wood and coil coatings, enabled by better resin design and coalescents. Moreover, regulatory pressure is spurring formulations that meet or exceed VOC limits: e.g. the California Air Resources Board promotes 10% solvent waterborne basecoats in automotive refinish.
Sustainability efforts include bio-based polymers, often blended or modified by film formation to reduce reliance on petrochemicals. At present, bio-based coatings are more common in non-critical applications (food packaging, paper coatings, furniture) but high-performance variants (e.g. partly soy-based wood finishes, vegetable-oil polyurethanes) are emerging. A key challenge is that many high-performance polymers still lack direct waterborne equivalents, so hybrid strategies (e.g. core–shell latex with petro outer layer and natural core) are an area of active research.
Conclusion
Film formation in coatings remains a critical interface of science and industry. The basic mechanisms are well understood, but controlling them in complex formulations is challenging. Key trends include a near complete industry shift to waterborne and high-solids systems for environmental compliance, alongside the pursuit of sustainable raw materials and low VOC additives. Advanced analytical tools are deepening the understanding of the film forming front. Future coatings are likely to feature even higher solids, novel polymer chemistries (waterborne polyurethanes, hybrid water/UV systems), and more bio‐based content. By tuning Tg/MFFT, additive synergies and drying processes, formulators continue to achieve high‐performance films while meeting regulatory and sustainability goals. The outlook is that film‐forming polymers in coatings will evolve to offer ever‐better functional properties (durability, self‐cleaning, low‐temperature cure) in environmentally friendly formulations – the fundamental step of creating a continuous, defect‐free polymer film will remain at the heart of this innovation.
En rapport
!["Construction worker applying Scott Bader structural adhesives on rooftop with city skyline in background" [G]](https://a.storyblok.com/f/329344/4444x3333/56cbf2d783/industrial-coating.jpeg/m/320x400)
Épaississants à émulsion inverse
An overview of inverse emulsions, their benefits and formulation guidelines.
!["Colorful mosaic pavement with irregularly shaped stone tiles in green, yellow, red, and black, creating a textured decorative surface." [G]](https://a.storyblok.com/f/329344/5376x3584/f52a2f6956/tiles.jpeg/m/320x400)
Epaississants pour émulsions
Présentation générale des émulsions, directives de formulation et efficacité rhéologique.
!["Vibrant, glossy gelcoats in yellow, blue, red, and green colors showcasing Scott Bader's high-performance pigmented resins" [G]](https://a.storyblok.com/f/329344/5824x3264/64f0be4d65/paint.jpeg/m/320x400)
Technologie du liant
A closer look at the polymer chemistry behind Scott Bader's binder technology
